Cervical & Lumbar Disc Replacement

An Alternative to Fusion
Patients have varied anatomy and other total disc replacement systems offer limited optionality. Centinel Spine’s revolutionary prodisc® total disc replacement platform advances patient care by providing total disc replacement products that allow Dr. Joel D. Siegal to intraoperatively select the ideal prodisc® implant design that’s best for the patient.
The treatment goal is to restore the normal dynamic function of the spine and to significantly reduce pain.
The function of the spine is restored through the mechanism of action of the device. Pain reduction is achieved through the re-establishment of the disc height and maintained by the prosthesis. The increase in height and the elimination of the herniated disc “opens” constricted nerve paths and the vertebral joints are restored to their physiological position.
Lumbar Fusion

Medacta is an international orthopaedics company specializing in the design and production of innovative orthopaedic products and the development of accompanying surgical techniques. Established in 1999 in Switzerland, Medacta’s products and surgical techniques are characterized by innovation.
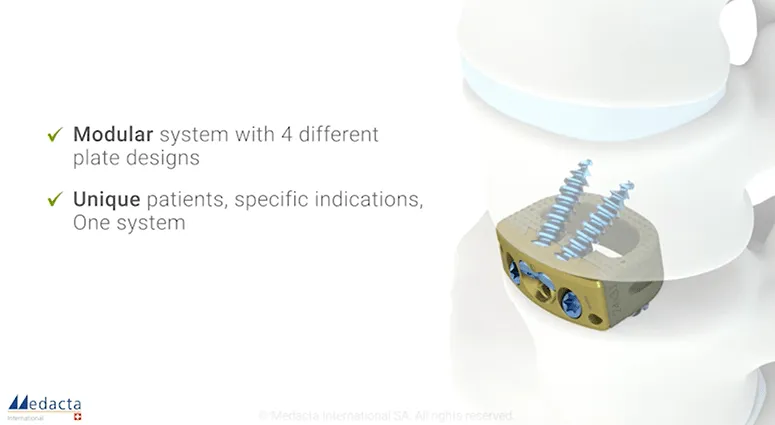
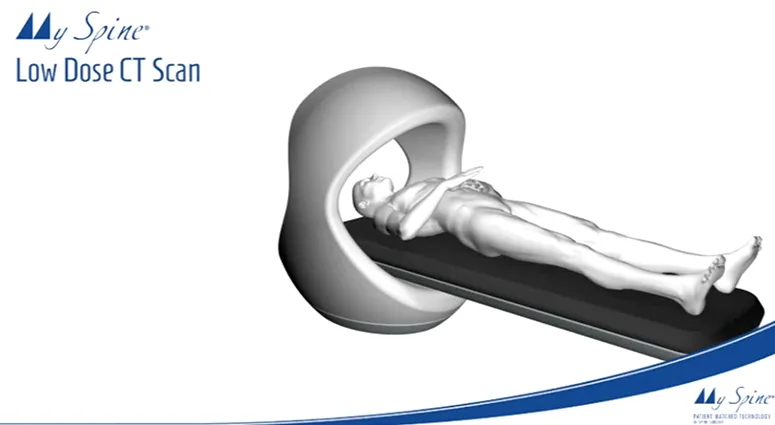
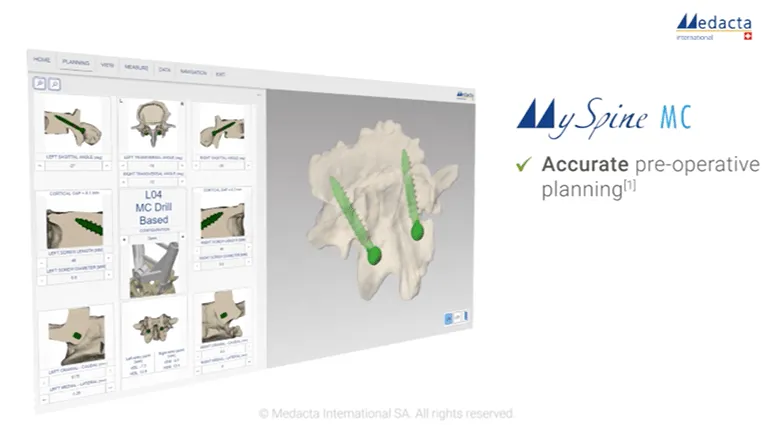
Orthofix's Firebird™ SI Fusion System
Introducing the revolutionary Orthofix's Firebird™ SI Fusion System, a cutting-edge solution for sacroiliac joint dysfunction. This family of 3D-printed titanium implants is designed to deliver optimal stabilization and compression of the sacroiliac joint throughout the fusion process.
The Firebird™ SI Fusion System represents a significant leap forward in spinal care, promising a new era of pain relief and stability for those suffering from sacroiliac joint issues.
Endoscopic Carpal Tunnel Release

The Seg-WAY™ endoscopic release system is a minimally invasive single-portal system providing quicker return to work, less patient scarring, and less postoperative pain. The system is used for both upper and lower extremity in Endoscopic Carpal Tunnel, Cubital Tunnel, Plantar Fasciitis and Gastrocnemius Releases.
Some of the benefits include:
Safety First
The system provides the ability to visualize fibers before release
Versatility
Includes different size guides for patient specificity
Assurance & Confidence
The surgeon can probe the fibers to ensure a complete release
Herniated Disc Hole Closure
Barricaid is a small implant, slightly larger than a pencil eraser that is designed to plug the larger holes in the disc wall. It is made up of a titanium bone anchor which secures the polymer plug into the disc space to repair and reconstruct disc wall.
Dr. Siegal has achieved the distinction of Barricaid Gold Center of Excellence. This designation honors consistent clinical excellence and a sustained commitment to optimizing outcomes for high-risk discectomy patients with Barricaid, which has been shown to reduce reoperations for reherniations by 81%*.

